Dengue fever is an acute mosquito-borne viral disease causes flu-like illness, which occasionally develops into a potential lethal complication called severe dengue (haemorrhagic) fever.
The causative agents are dengue viruses consisting of 4 serotypes. The viruses are transmitted to human through Aedes aegypti mosquitoes. About half of the world's population, especially South Asia and South America, is at risk.
One recent study indicated that there are 390 million dengue infections per year. Unfortunately, there is no specific treatment. Prevention, through Dengue vaccine, would be the best way to prevent dengue virus infection.
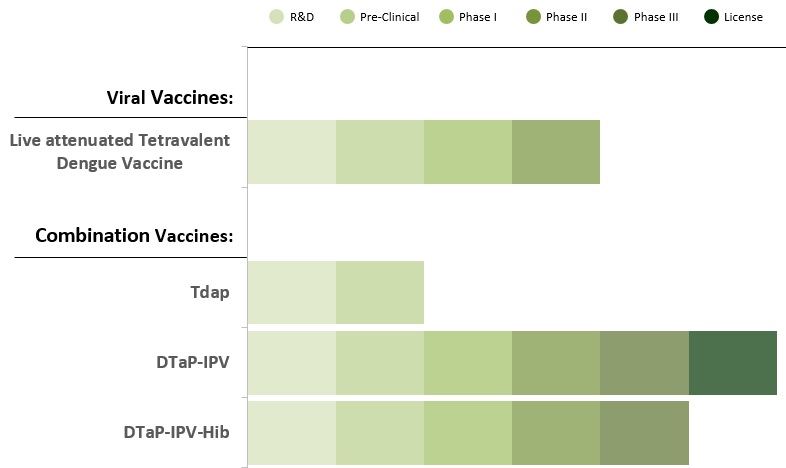
Diphtheria, Tetanus and Pertussis DTP combination has been used as the backbone for combination vaccines development with additional antigens such as Inactivated poliovirus and Haemophilus influenzae type b. This enables multiple vaccine antigens delivery via a single injection. Combining vaccines into fewer shots would mean on time protection with less pain and discomfort.
Two forms of pertussis vaccine are in use, the whole-cell vaccine (wP), and the acellular vaccine (aP). Immunization with wP containing vaccines has been frequently associated with adverse reactions, which increase with age and number of vaccinations injections. wP containing vaccines are therefore not recommended for adolescents and adults immunization.
To address the adverse reactions observed with wP vaccines, aP vaccines containing purified components of B. Pertussis inactivated pertussis toxin (PT), either alone or in combination with filamentous haemagglutinin (FHA), fimbrial antigens (FIM2/3) and pertactin (PRN) were developed.
DTP-containing multi-antigen vaccines (with IPV, Hib) are commonly included in national immunization campaigns for children.
Reduced diphtheria, pertussis and tetanus vaccine (Tdap) is recommended for adolescents at age 11 or 12 years, pregnant women to protect their newborn baby and adults as booster dose every 10 years.